Investigate PhosphoSens technology for precise kinase and phosphatase activity measurement
Explore how PhosphoSens® technology offers a solution to precisely measure kinase and phosphatase activity, helping researchers advance their understanding of cellular signaling and therapeutic development.

Protein phosphorylation and dephosphorylation are fundamental biochemical processes that regulate cellular functions, including signal transduction, cell cycle progression, metabolism, and gene expression. These processes are catalyzed by two classes of enzymes: kinases and phosphatases.
Protein kinases are enzymes that play a critical role in regulating cellular functions (Cheng et al., 2011). These enzymes are involved in processes such as cell growth, differentiation, metabolism, proliferation, and programmed cell death (Shchemelinin et al., 2006). The human genome encodes 518 different protein kinases, making them one of the largest families of enzymes in the body (Manning et al., 2002). Due to their crucial role in cellular signaling, disruptions in kinase activity are linked to many diseases, including cancer and other conditions.
Protein phosphatases are enzymes that play a crucial role in cellular regulation by removing phosphate groups from phosphorylated amino acid residues on proteins. They are classified into several families based on their structure and catalytic mechanisms.
To better understand protein kinases and phosphatase and develop effective therapies, it is important to precisely measure their activity. This is where tools like the AssayQuant’s PhosphoSens Kinase and Phosphatase Assays come in. These platform allows for accurate and sensitive detection of kinase and phosphatase activity, which is essential for both basic research and therapeutic development.
PhosphoSens® Technology
The core of PhosphoSens® technology relies on the Chelation-Enhanced Fluorescence (ChEF) method. This method uses a synthetic amino acid with an 8-hydroxyquinoline derivative (sulfonamidooxine, Sox). Sox binds to magnesium ions (Mg²⁺) and interacts with the phosphorylation sites on the kinase substrates.
In a non-phosphorylated state, Sox has low affinity for Mg²⁺, meaning there is minimal fluorescence. However, once the substrate is phosphorylated, Sox’s affinity for Mg²⁺ increases, resulting in a strong fluorescence signal. This fluorescence can then be monitored using standard fluorescence plate readers, making it easy to quantify kinase activity (Figure 1).
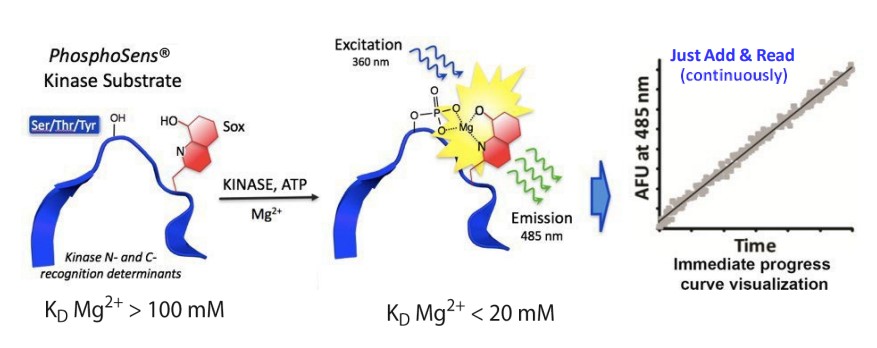
Figure 1: ChEF Mechanism for Direct Protein Kinase Activity Sensing
Figure 2A illustrates typical fluorescence changes upon phosphorylation of a PhosphoSens peptide substrate and Figure 2B shows excitation and emission spectra of a typical phosphorylated PhosphoSens peptide. The fluorescence properties of the Mg(II)-coordinated with the 8- hydroxyquinoline of Sox has an λExMax of ~360 nm (358-363 nm) and λEmMax of ~492 nm (485-498 nm). Since the fluorescence emission spectrum is relatively broad (see Figure 2B), fluorescence emission can be monitored between 475-508 nm with <7% loss in signal intensity.
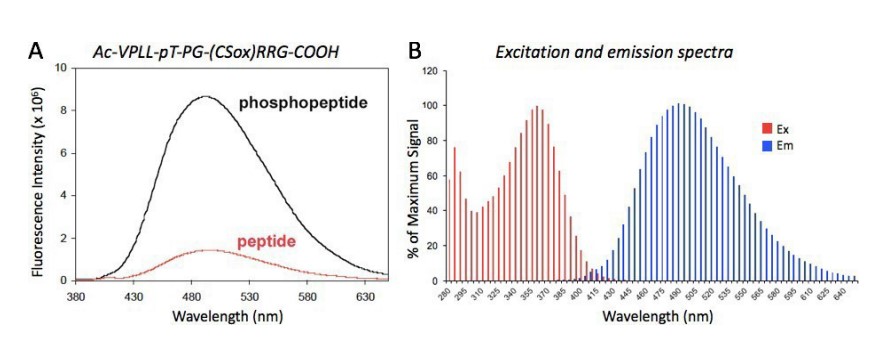
Figure 2: Fluorescence Spectra of PhosphoSens Peptides
PhosphoSens® Kinase and Phosphatase
PhosphoSens® kits, which include CSox peptide substrates, are compatible with various kinase types, both purified and those found in complex biological samples. This flexibility makes PhosphoSens® an excellent tool for diverse research applications, from kinase inhibitor screening to analyzing the impact of kinase mutations.
For phosphatase assays, PhosphoSens® employs a similar approach, using CSox-based phosphopeptides. These assays are also homogeneous, continuous, and highly sensitive, providing reliable data for studying phosphatase activity in physiological settings.
PhosphoSens® provides a simple yet powerful solution for measuring kinase and phosphatase activity in a variety of biological contexts. Whether you're studying kinase inhibitors, analyzing kinase mutations, or exploring phosphatase regulation, PhosphoSens® offers reliable, efficient, and accurate results. By simplifying these complex measurements, PhosphoSens® is helping researchers unravel the mysteries of cellular signaling and paving the way for the development of targeted therapeutic agents.
References
Cheng, H.C., Qi, R.Z., Paudel, H. and Zhu, H.J., 2011. Regulation and function of protein kinases and phosphatases. Enzyme research, 2011, p.794089.
Shchemelinin, I., Sefc, L. and Necas, E., 2006. Protein kinases, their function and implication in cancer and other diseases. Folia biologica, 52(3), p.81.
Manning, G., Whyte, D.B., Martinez, R., Hunter, T. and Sudarsanam, S., 2002. The protein kinase complement of the human genome. Science, 298(5600), pp.1912-1934.

