CRISPR-Cas Systems: Revolutionizing Genome Editing Technology
CRISPR-Cas9: Unlocking precision genome editing for breakthroughs in medicine, agriculture, and beyond.

The discovery of CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) and its associated Cas proteins has democratized genome editing, offering unprecedented precision and versatility. Pioneered by the foundational work of Doudna and Charpentier (2012), CRISPR-Cas9 harnesses a bacterial defense mechanism to create targeted double-strand DNA breaks, enabling efficient gene knockout or knock-in via homology-directed repair (HDR). Since then, CRISPR systems have diversified, with innovations such as base editors, prime editors, and CRISPR interference (CRISPRi/a) expanding the toolbox for precision genome engineering.
CRISPR-Cas systems operate via a two-component mechanism where guide RNA (gRNA) directs the Cas9 endonuclease to a specific genomic location, leading to double-strand breaks (DSBs) (Zakrzewska and Burmistrz, 2023). These breaks are then repaired either through non-homologous end joining (NHEJ) or homology-directed repair (HDR), enabling precise gene editing (Zakrzewska and Burmistrz, 2023). The entire process can be divided into three stages: recognition, cleavage, and repair (Asmamaw and Zawdie, 2021). Initially, the Cas9: sgRNA complex binds to DNA (Bhattacharya and Satpati, 2022). Mature crRNA is incorporated into the effector complex and searches for sequences complementary to its spacer fragment (Zakrzewska and Burmistrz, 2023). For Class 2 Type II systems, the Cas9 protein complex, along with mature crRNA and tracrRNA, scans DNA for a protospacer adjacent motif (PAM) (Zakrzewska and Burmistrz, 2023). Recognition of the complementary sequence induces an R loop structure, leading to a double-strand cleavage via the HNH and RuvC domains (Zakrzewska and Burmistrz, 2023).
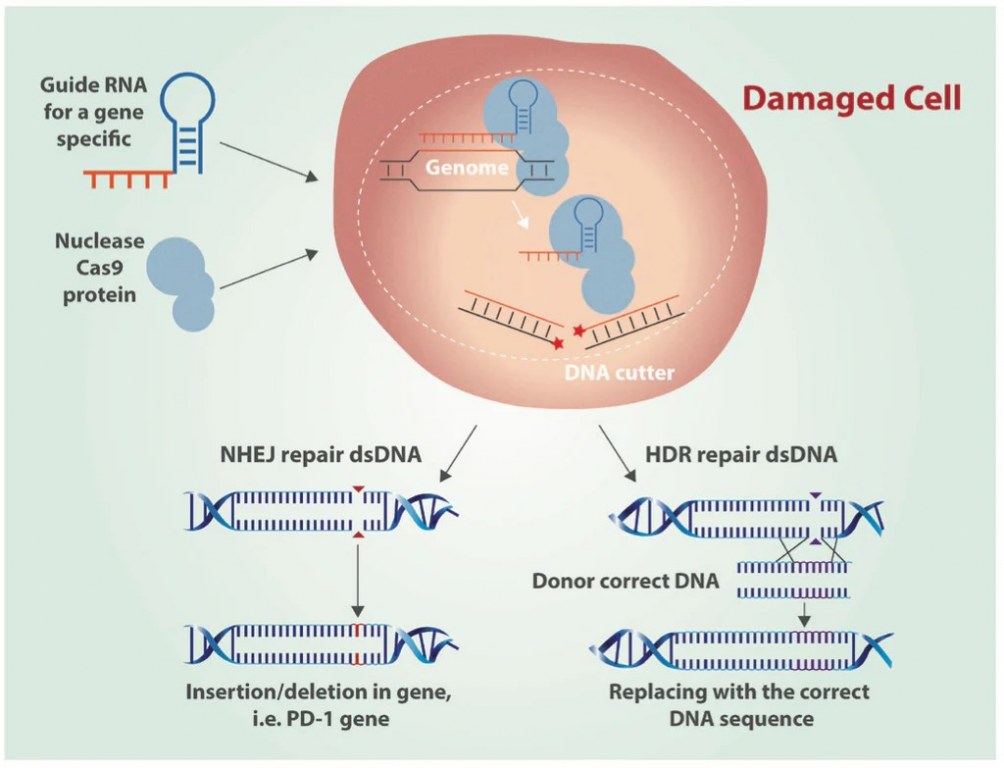
Figure 1: Gene editing by CRISPR Cas9 DNA using non-homologus end joining (NHEJ) and homology-directed repair (HDR) (Castillo, 2016).
Applications
Gene Therapy
One of the most promising applications of CRISPR-Cas technology lies in gene therapy. By directly targeting genetic mutations responsible for diseases, researchers can potentially correct these defects at the source. Notable successes include the editing of the CCR5 gene in human T cells to confer resistance to HIV infection (Zhu, 2022). Moreover, CRISPR has been utilized to develop mouse models that mimic human diseases, facilitating the study of pathophysiological mechanisms and therapeutic strategies (Cribbs and Perera, 2017).
Precision Medicine
CRISPR's ability to edit genes with precision opens new avenues for personalized medicine. By tailoring treatments based on an individual's genetic makeup, CRISPR can enhance therapeutic efficacy while minimizing adverse effects. For instance, ongoing research is exploring its application in cancer therapy by targeting oncogenes and tumor suppressor genes to inhibit tumor growth (Zhu, 2022).
Agricultural Biotechnology
Beyond human health, CRISPR-Cas systems are revolutionizing agricultural practices. The technology enables the development of crops with enhanced traits such as disease resistance, drought tolerance, and improved nutritional profiles. This biotechnological advancement promises to address food security challenges posed by climate change and population growth (Zhu, 2022).
CRISPR-Cas systems represent a groundbreaking advancement in genetic engineering with far-reaching implications across various fields. As research continues to unveil the full potential of this technology, it is imperative that scientists engage in responsible practices that prioritize safety and ethical considerations. The future of precision biology hinges on our ability to harness these powerful tools while navigating the complexities they present.
In summary, CRISPR-Cas technology stands at the forefront of scientific innovation, offering transformative solutions for genetic diseases and agricultural challenges alike. Continued exploration and dialogue surrounding its applications will be crucial as we move towards a future where precise genetic editing becomes a standard practice in medicine and biotechnology.
References
Doudna, J.A. and Charpentier, E., 2014. The new frontier of genome engineering with CRISPR-Cas9. Science, 346(6213), p.1258096.
Zakrzewska, M. and Burmistrz, M., 2023. Mechanisms regulating the CRISPR-Cas systems. Frontiers in Microbiology, 14, p.1060337.
Asmamaw, M. and Zawdie, B., 2021. Mechanism and applications of CRISPR/Cas-9-mediated genome editing. Biologics: targets and therapy, pp.353-361.
Bhattacharya, S. and Satpati, P., 2022. Insights into the mechanism of CRISPR/Cas9-based genome editing from molecular dynamics simulations. ACS omega, 8(2), pp.1817-1837.
Castillo, A., 2016. Gene editing using CRISPR-Cas9 for the treatment of lung cancer. Colombia Médica, 47(4), pp.178-180.
Zhu, Y., 2022. Advances in CRISPR/Cas9. BioMed research international, 2022(1), p.9978571.
Cribbs, A.P. and Perera, S.M., 2017. Focus: Genome editing: Science and bioethics of CRISPR-Cas9 gene editing: An analysis towards separating facts and fiction. The Yale journal of biology and medicine, 90(4), p.625.

