CGP 55845 hydrochloride [149184-22-5]
Cat# B5086-10mg
Size : 10mg
Brand : APExBIO Technology
Request more information
Please log in to use this feature.
CGP 55845 hydrochloride
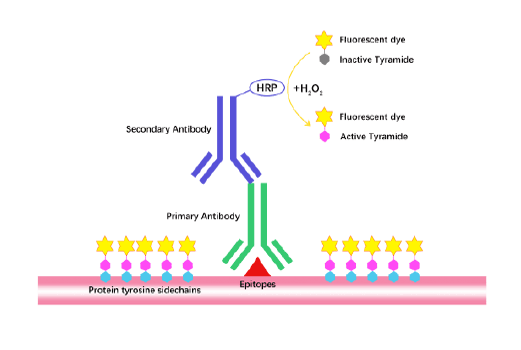
Tyramide Signal Amplification (TSA)
TSA (Tyramide Signal Amplification), used for signal amplification of ISH, IHC and IC etc.
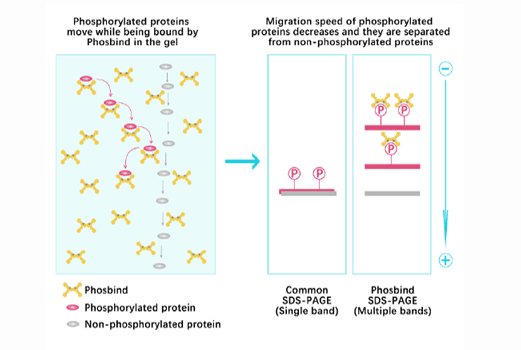
Phos Binding Reagent Acrylamide
Separation of phosphorylated and non-phosphorylated proteins without phospho-specific antibody
Background
IC50: 5 nM
CGP 55845 is a potent, selective GABAB receptor antagonist that abolishes agonist binding (pKi = 8.35) and blocks GABA and glutamate release (pEC50 values are 8.08 and 7.85 respectively). CGP 55845 prevents GABAB responses to baclofen (IC50 = 130 nM in an isoproterenol assay) and increases the hypoglycemic response to glucose in vitro. [1,2]
Presynaptic GABAB receptors seem to regulate the release of several neurotransmitters. Baclofen, the GABAB agonist, that prevents the release of GABA itself via autoreceptors, was 10 times more potent in antagonizing the inhibitory effect of (-)-baclofen on the release of GABA and of somatostatin-like immunoreactivity (SRIF-LI) than of glutamate. However, CGP 35348 was about 70 times more potent in preventing the effect of baclofen on glutamate and SRIF-LI than on GABA release.
In vitro: Antagonist CGP 55845A of the GABAB receptor in the presence of CNQX and d(2)-2-amino-5-phosphonovaleric acid prevented the inhibitory postsynaptic potential-B and paired-pulse depression. [3].
This secretion was cadmium sensitive, potentiated by CGP 55845, and blocked by ketanserin. Taken together these data suggest that CB receptors act as direct glucosensors, and that processing of hypoglycaemia utilizes similar neurotransmitter and neuromodulatory mechanisms as hypoxia [4]. The convulsant 4-aminopyridine (4-AP) and the GABAB receptor antagonist CGP 55845 both applied to adult guinea pig hippocampal to slices, resulting in eliciting giant GABA-mediated postsynaptic potentials (GPSPs) and epileptiform discharges. [5].
In vivo: So far, no study in vivo has been conducted.
Clinical trial: So far, no clinical study has been conducted.
References:
[1] Waldmeier PC, Wicki P, Feldtrauer JJ, Mickel SJ, Bittiger H, Baumann PA. GABA and glutamate release affected by GABAB receptor antagonists with similar potency: no evidence for pharmacologically different presynaptic receptors. Br J Pharmacol. 1994 Dec;113(4):1515-21.
[2] Cunningham MD, Enna SJ. Evidence for pharmacologically distinct GABAB receptors associated with cAMP production in rat brain. Brain Res. 1996 May 13;720(1-2):220-4.
[3] Deisz RA. The GABA(B) receptor antagonist CGP 55845A reduces presynaptic GABA(B) actions in neocortical neurons of the rat in vitro. Neuroscience. 1999;93(4):1241-9.
[4] Zhang M, Buttigieg J, Nurse CA. Neurotransmitter mechanisms mediating low-glucose signalling in cocultures and fresh tissue slices of rat carotid body. J Physiol. 2007 Feb 1;578(Pt 3):735-50. Epub 2006 Nov 23.
[5] Salah A, Perkins KL. Effects of subtype-selective group I mGluR antagonists on synchronous activity induced by 4-aminopyridine/CGP 55845 in adult guinea pig hippocampal slices. Neuropharmacology. 2008 Jul;55(1):47-54. doi: 10.1016/j.neuropharm.2008.04.010. Epub 2008 Apr 23.
Product Citation
Chemical Properties
| Physical Appearance | White solid |
| Storage | Store at RT |
| M.Wt | 438.71 |
| Cas No. | 149184-22-5 |
| Formula | C18H22Cl2NO3Pmg/ml in DMSO |
| Chemical Name | benzyl((S)-3-(((S)-1-(3,4-dichlorophenyl)ethyl)amino)-2-hydroxypropyl)phosphinic acid hydrochloride |
| SDF | Download SDF |
| Canonical SMILES | C[C@@](NC[C@@](O)(05)CP(CC1=CC=CC=C1)(O)=O)(05)C2=CC(Cl)=C(Cl)C=C2.Cl |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |