Docetaxel [114977-28-5]
Cat# A4394-100mg
Size : 100mg
Brand : APExBIO Technology
Request more information
Please log in to use this feature.
Docetaxel
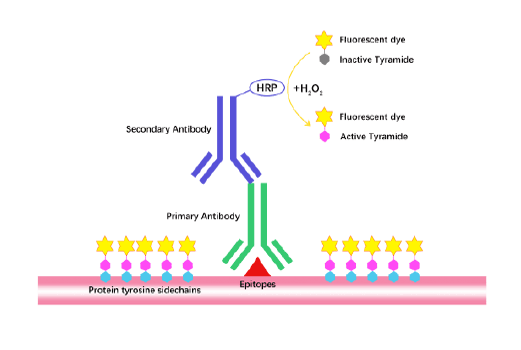
Tyramide Signal Amplification (TSA)
TSA (Tyramide Signal Amplification), used for signal amplification of ISH, IHC and IC etc.
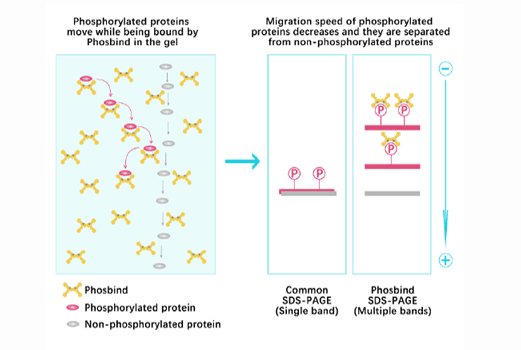
Phos Binding Reagent Acrylamide
Separation of phosphorylated and non-phosphorylated proteins without phospho-specific antibody
Background
Docetaxel, a new taxoid family member originally derived from the needles of the European Yew tree Taxus baccata, is a potent chemotherapeutic agent that acts as a spindle poison to inhibit microtubule dynamics and cell cycle arrest through promoting microtublin assembly and stabilizing the polymers against depolymerization. Docetaxel has demonstrated strong in vivo and in vitro antitumor activities against a broad range of cancers including breast, lung, ovarian, head and neck, and gastric cancers. Previous studies have shown that docetaxel exertss stronger cytotoxicity than other chemotherapeutic agents against ovarian carcinoma cell lines, in which the cytotoxicity of docetaxel is 1.2-2.6 times greater than that of paclitaxel and over 1000 times greater than that of cisplatin or etoposide.
Reference
N Katsumata. Docetaxel: an alternative taxane in ovarian cancer. British Journal of Cancer (2003) 89 (Suppl 3), S9-S15
Product Citation
- 1. Schwartz, Hannah, et al. "In vitro Methods to Better Evaluate Drug Responses in Cancer." UMass Chan Medical School. September 8, 2022.
- 2. Weibo Zhong, Kaihui Wu, et al. "Gut dysbiosis promotes prostate cancer progression and docetaxel resistance via activating NF-κB-IL6-STAT3 axis." Microbiome. 2022 Jun 16;10(1):94. PMID: 35710492
- 3. Mikhail S. Chesnokov, Marianna Halasi, et al. "Novel FOXM1 inhibitor identified via gene network analysis induces autophagic FOXM1 degradation to overcome chemoresistance of human cancer cells." Cell Death Dis. 2021 Jul 14;12(7):704. PMID: 34262016
- 4. Robert Craig Peery. "Optimization of Survivin Dimerization Inhibitors for the Treatment of Docetaxel-Resistant Prostate Cancer." January 2020.
- 5. Yan L, Ding B, et al. "Inhibition of SMYD2 suppresses tumor progression by down-regulating microRNA-125b and attenuates multi-drug resistance in renal cell carcinoma." Theranostics. 2019 Oct 22;9 (26):8377-8391. PMID: 31754403
- 6. Zhang Y, Xia F, et al. "miR-135b-5p enhances doxorubicin-sensitivity of breast cancer cells through targeting anterior gradient 2." J Exp Clin Cancer Res. 2019 Jan 21;38(1):26. PMID: 30665445
- 7. Li Q, Deng Q, et al. "Linking prostate cancer cell AR heterogeneity to distinct castration and enzalutamide responses." Nat Commun. 2018 Sep 6;9(1):3600. PMID: 30190514
- 8. Zhou XW, Xia YZ, et al. "Tomentodione M sensitizes multidrug resistant cancer cells by decreasing P-glycoprotein via inhibition of p38 MAPK signaling. Oncotarget." 2017 Oct 19;8(60):101965-101983. PMID: 29254218
Chemical Properties
| Physical Appearance | A solid |
| Storage | Store at -20 |
| Cas No. | 114977-28-5 |
| Formula | C43H53NO14 |
| Synonyms | Taxotere, Docetaxel anhydrous, Trihydrate |
| Solubility | mg/mL in DMSO; insoluble in H2O; mg/mL in EtOH |
| SDF | Download SDF |
| Canonical SMILES | O=C(N[C@H]([C@H](C(O[C@H]1C[C@]2(O)C(C)(C)C([C@@H](O)C([C@@]3(C)[C@]([C@@](CO4)(OC(C)=O)[C@@]4(10)C[C@@H]3O)(10)[C@@H]2OC(C5=CC=CC=C5)=O)=O)=C1C)=O)O)C6=CC=CC=C6)OC(C)(C)C |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Protocol
| Cell experiment [1]: | |
| Cell lines | Nine human gastric cancer cell lines |
| Preparation method | The solubility of this compound in DMSO is > 10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20 012 ~ > 1.2 μM |
| Applications | The cytotoxic effect of Docetaxel was relatively greater than that of Paclitaxel in six of the nine cells. The effect of Docetaxel and Paclitaxel on MM-7 and ST-SA-I cells was less than on the other seven cultured cells. |
| Animal experiment [1]: | |
| Animal models | Mice bearing human gastric cancer xenografts (MKN-28, MKN-45 and KKLS) |
| Dosage form | 3.75, 7.5, 15 or 22 mg/kg; i.v.; three times within a 4-day interval |
| Applications | Docetaxel dose-dependently inhibited tumour growth. At the doses of 15 and 22 mg/kg, Docetaxel induced complete tumor regression in all mice. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1]. Tanaka M, Obata T, Sasaki T. Evaluation of antitumour effects of docetaxel (Taxotere) on human gastric cancers in vitro and in vivo. Eur J Cancer. 1996 Feb;32A(2):226-30. | |
Biological Activity
| Description | Docetaxel, an analog of taxol, is an inhibitor of depolymerisation of microtubules by binding to stabilized microtubules. | |||||
| Targets | Microtubules | |||||
| IC50 | ||||||
Quality Control
Related Biological Data

Related Biological Data

Related Biological Data

Related Biological Data

Related Biological Data