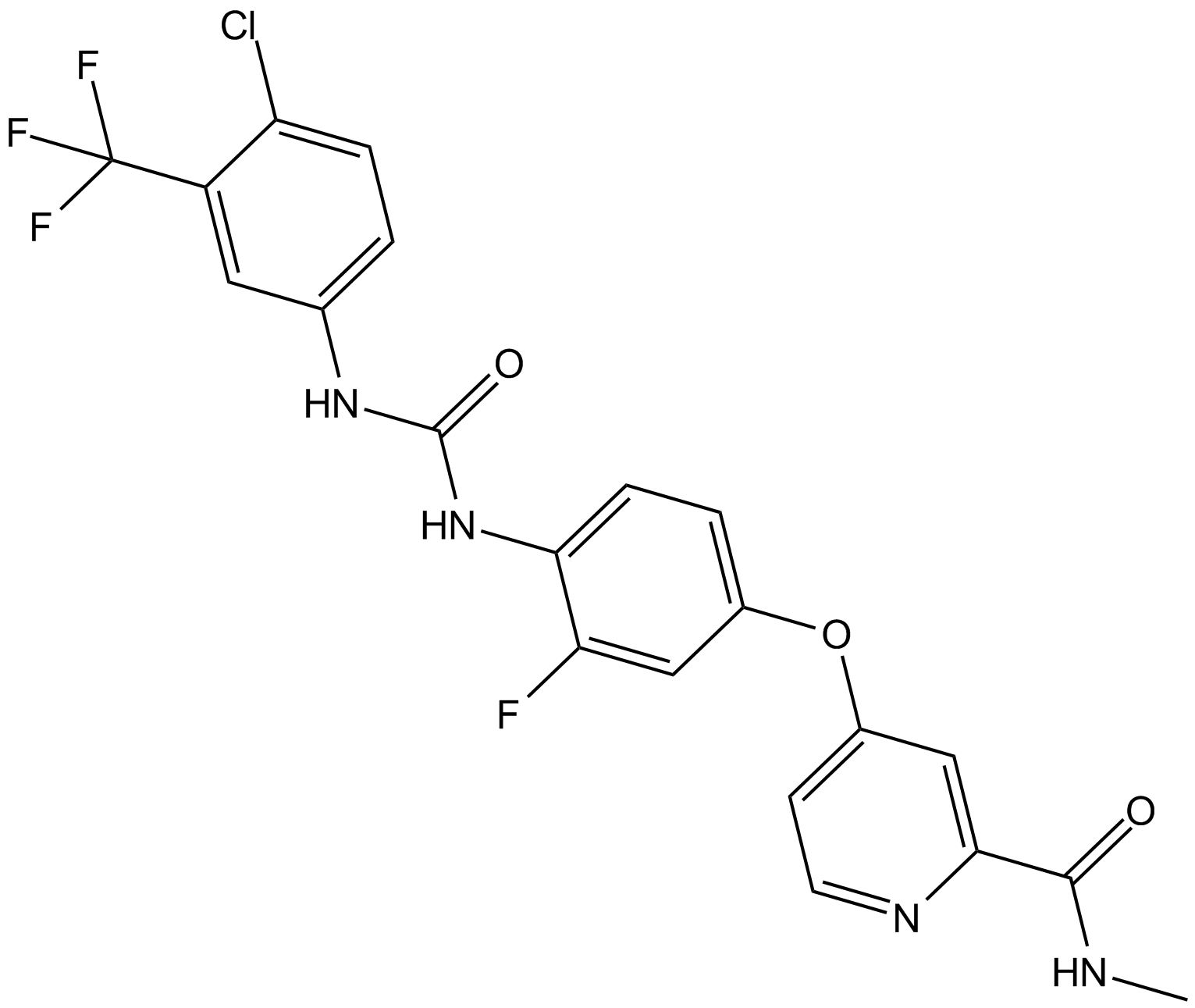
Regorafenib [755037-03-7]
Cat# A8236-10mg
Size : 10mg
Brand : APExBIO Technology
Request more information
Please log in to use this feature.
Regorafenib
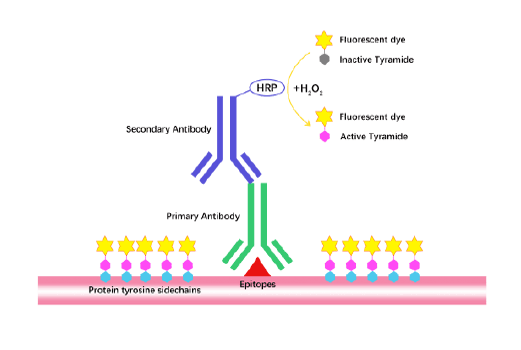
Tyramide Signal Amplification (TSA)
TSA (Tyramide Signal Amplification), used for signal amplification of ISH, IHC and IC etc.
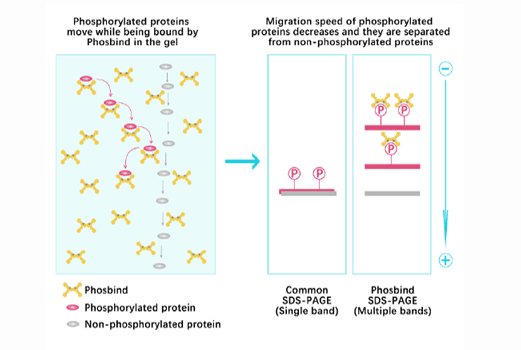
Phos Binding Reagent Acrylamide
Separation of phosphorylated and non-phosphorylated proteins without phospho-specific antibody
Background
Regorafenib (BAY 73-4506) is a novel and orally active multikinase inhibitor of receptor tyrosine kinases VEGFR1, VEGFR2, VEGFR3, PDGFRβ, Kit, RET, Raf-1, B-RAF and B-RAFV600E with IC50 values of 13 nM/4.2 nM/46 nM, 22 nM, 7 nM, 1.5 nM, 2.5 nM, 28 nM and 19 nM [1].
VEGFR1/2/3 are vascular endothelial growth factor receptor plays an important role in the formation of normal and tumor vasculature. platelet-derived growth factor receptor-β (PDGFRβ) is a receptor for the platelet-derived growth factor family. Kit, RET and B-RAF are both receptor tyrosine kinases that encoded by proto-oncogenes [1].
Regorafenib (BAY 73-4506) is a novel and orally active receptor tyrosine kinases inhibitor. In NIH-3T3/VEGFR2 cells, regorafenib potently inhibited VEGFR2 autophosphorylation with IC50 value of 3 nM. Regorafenib also inhibited TIE2 and PDGFR-β autophosphorylation with IC50 values of 31 and 90 nM. Regorafenib potently inhibited KITK642E and RETC634W with IC50 values of ~20 and ~10 nM, respectively. In addition, regorafenib inhibited the proliferation of VEGF165-stimulated HUVECs with IC50 value of ~3 nM [1].
In GS9L glioblastoma xenografted rat model, regorafenib administered orally at 10 mg/kg significantly reduced the extravasation of Gadomer in the vasculature. In various preclinical human xenograft mice models, regorafenib exhibited potent dose-dependent tumor growth inhibition (TGI) [1]. In murine metastatic colorectal cancer (CRC) liver metastasis model, regorafenib significantly delayed disease progression by inhibiting the growth of liver metastases and preventing the formation of new metastases in other organs [2].
References:
[1]. Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer, 2011, 129(1): 245-255.
[2]. Schmieder R, Hoffmann J, Becker M, et al. Regorafenib (BAY 73-4506): antitumor and antimetastatic activities in preclinical models of colorectal cancer. Int J Cancer, 2014, 135(6): 1487-1496.
Product Citation
- 1. ZI‑YI CHEN, JIE LI, et al. "Harmine reinforces the effects of regorafenib on suppressing cell proliferation and inducing apoptosis in liver cancer cells." Exp Ther Med. 2022 Mar;23(3):209. PMID:35126712
- 2. Barot S, Abo-Ali EM, et al. "Inhibition of glycogen catabolism induces intrinsic apoptosis and augments multikinase inhibitors in hepatocellular carcinoma cells." Exp Cell Res. 2019 Aug 15;381(2):288-300. PMID:31128107
- 3. Wu LW, Zhou DM, et al. "Suppression of LSD1 enhances the cytotoxic and apoptotic effects of regorafenib in hepatocellular carcinoma cells." Biochem Biophys Res Commun. 2019 May 14;512(4):852-858. PMID:30929918
- 4. Hu X, Wu LW, et al. "The anti-tumor effect of regorafenib in lung squamous cell carcinoma in vitro." Biochem Biophys Res Commun. 2018 Sep 5;503(2):1123-1129. PMID:29944884
- 5. Yang Q, Guo X, et al. "Metformin Enhances the Effect of Regorafenib and Inhibits Recurrence and Metastasis of Hepatic Carcinoma After Liver Resection via Regulating Expression of Hypoxia Inducible Factors 2α (HIF-2α) and 30 kDa HIV Tat-Interacting Protein (TIP30)." Med Sci Monit. 2018 Apr 14;24:2225-2234. PMID:29654226
- 6. Zhang WJ, Li Y, et al. "Synergistic antitumor activity of regorafenib and lapatinib in preclinical models of human colorectal cancer." Cancer Lett. 2017 Feb 1;386:100-109. PMID:27864115
Chemical Properties
| Physical Appearance | A solid |
| Storage | Desiccate at -20°C |
| M.Wt | 482.82 |
| Cas No. | 755037-03-7 |
| Formula | C21H15ClF4N4O3 |
| Synonyms | BAY 73-4506 |
| Solubility | ≥25.04 mg/mL in DMSO; insoluble in H2O; ≥6.25 mg/mL in EtOH with ultrasonic |
| Chemical Name | 4-(4-(3-(4-chloro-3-(trifluoromethyl)phenyl)ureido)-3-fluorophenoxy)-N-methylpicolinamide |
| SDF | Download SDF |
| Canonical SMILES | O=C(NC)C1=CC(OC2=CC=C(NC(NC3=CC=C(Cl)C(C(F)(F)F)=C3)=O)C(F)=C2)=CC=N1 |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |
Protocol
| Cell experiment: [1] | |
| Cell lines | PLC/PRF/5 cells |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
| Reaction Conditions | 1 μM, 72 hours for migration assay 5 μM, 24 hours for invasion assay |
| Applications | For the migration assay, PLC/PRF/5 cells were treated with the drugs and microscopically analyzed at the time of the scratch (T0) and after 48 and 72 hours. For the invasion assay, invading PLC/PRF/5 cells were treated with different drug concentrations (0.5, 1, 2.5 and 5 μM). Invasion was calculated as a percentage of the invading drug-treated cells compared to drug-untreated control cells. Regorafenib inhibited HCC cell migration in both AFP-positive and AFP-negative cells at the same low concentration range as inhibited AFP levels. Similar results were found in a cell invasion assay, at almost identical drug concentrations. |
| Animal experiment: [2] | |
| Animal models | Female athymic NCr nu/nu mice injected with Colo-205, MDA-MB-231 or 786-O xenografts |
| Dosage form | Oral administration; 100, 30, 10, and 3 mg/kg |
| Applications | Regorafenib dosed qd orally inhibited tumor growth in a dose-dependent manner in multiple xenograft models, including models derived from CRC (Colo-205), BC (MDA-MB-231) and RCC (786-O) tumors. Regorafenib effectively inhibited growth of the Colo-205 xenografts in the dose range of 10-100 mg/kg, reaching a TGI of about 75% at day 14 at the 10 mg/kg dose. A slow regrowth was observed at all doses when treatment was terminated after 9 days. In the MDA-MB-231 model, regorafenib was highly efficacious at a dose as low as 3 mg/kg, resulting in a significant TGI of 81%, which increased to ~ 93% at doses of 10 and 30 mg/kg, where tumor stasis was reached. Regorafenib also very efficiently inhibited the growth of the 786-O RCC model. TGI >90% was observed at the end of a 21-day dosing period with regorafenib 10 and 30 mg/kg. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1] Carr B I, D'Alessandro R, Refolo M G, et al. Effects of low concentrations of regorafenib and sorafenib on human HCC cell AFP, migration, invasion, and growth in vitro. Journal of cellular physiology, 2013, 228(6): 1344-1350. [2] Wilhelm S M, Dumas J, Adnane L, et al. Regorafenib (BAY 73‐4506): A new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. International Journal of Cancer, 2011, 129(1): 245-255. | |
Biological Activity
| Description | Regorafenib (BAY 73-4506) is a multi-target inhibitor of VEGFR1, VEGFR2, VEGFR3, PDGFRβ, Kit, RET and Raf-1 with IC50 of 13 nM/4.2 nM/46 nM, 22 nM, 7 nM, 1.5 nM and 2.5 nM, respectively. | |||||
| Targets | VEGFR1/2/3 | PDGFRβ | Kit | RET | Raf-1 | |
| IC50 | 13 nM/4.2 nM/46 nM | 22 nM | 7 nM | 1.5 nM | 2.5 nM | |
Quality Control
Related Biological Data

Related Biological Data

Related Biological Data

Related Biological Data

Related Biological Data

Related Biological Data